Abstract
Tissue engineering seeks to create functional tissues and organs by integrating natural or synthetic scaffolds with cells and bioactive factors. Creating biologically active scaffolds that support key aspects of tissue regeneration, including the re-establishment of a functional extracellular matrix (ECM), is a challenge currently facing this field. During tissue repair, fibronectin is converted from an inactive soluble form to biologically active ECM fibrils through a cell-dependent process.
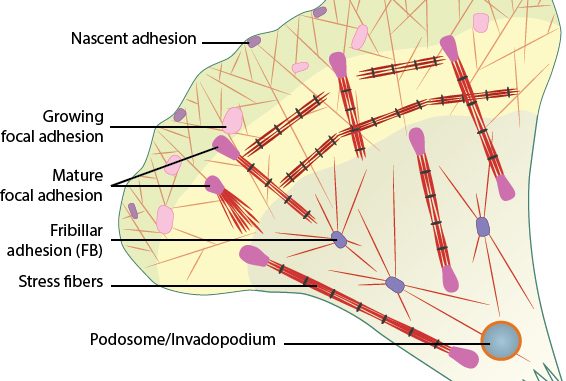
ECM fibronectin promotes cellular processes critical for tissue regeneration and regulates the deposition and organization of other ECM proteins. Previously, we developed fibronectin ECM biomimetics by directly coupling the heparin-binding fragment of the first fibronectin type III repeat (FNIII1H) to the integrin-binding repeats (FNIII8-10). As adhesive substrates, fibronectin matrix mimetics promote cell growth, migration, and contractility through an FNIII1H-dependent mechanism.
Here, we screened fibronectin matrix mimetic variants engineered to include all or part of the integrin-binding domain for their ability to support new ECM assembly. We found that specific modifications of the integrin-binding domain produced adhesive substrates that selectively coupled to different integrin receptors to, in turn, regulate the amount of fibronectin and collagen deposited in the ECM. The ability of fibronectin matrix mimetics to direct cell-substrate interactions and regulate ECM assembly makes them promising candidates for use as bioactive surfaces, where precise control over integrin-binding specificity and cell binding is required. ECM deposition.
Materials And Methods
- Cell lines
Cell lines used included A10 rat aortic smooth muscle cells (Kimes and Brandt, 1976a), A549 human lung carcinoma cells, C2C12 mouse skeletal myoblasts (Blau et al., 1985), C32 human melanoma cells, monkey kidney COS-7 (Gluzman, 1981), human melanoma cells G361, mouse skeletal myoblasts G8 (Christian et al., 1977), rat myoblasts H9c2 (Kimes and Brandt, 1976b), human fibrosarcoma HT1010 ( Rasheed et al., 1974), canine kidney MDCK cells, human osteosarcoma cells MG-63, human bladder carcinoma cells RT4, and human neuroblastoma cells SK-N-SH (Spengle et al., 1973). Most cell lines were grown in DMEM containing 10% fetal calf serum; however, the myoblast cell lines were grown in DMEM containing 20% fetal calf serum. All cells were kept in a humidified atmosphere with 10% CO2 at 37°C.
- Gel Electrophoresis and Western Blot
To examine fascin expression in a panel of cell lines, growing cultures containing 5 × 10 5 cells per plate were lysed directly in an SDS-PAGE sample buffer containing 100 mM dithiothreitol. SDS-PAGE was carried out according to the method of Laemmli (1970) on 12.5% polyacrylamide gels. Proteins were transferred to nitrocellulose (0.22 μm, pore size, Bio-Rad, Watford, UK) at 60 V for 18 h, using a Transblot apparatus (Bio-Rad) and a blot buffer composed of a base of 25 mM Tris(hydroxymethyl)aminomethane, 92 mM glycine, and 20% methanol (Towbin et al., 1979).
Non-specific binding sites were blocked by incubation overnight at 4°C in TBS containing 2% bovine serum albumin and 0.05% Tween 20. for 2 h at room temperature with vigorous shaking, washed three times for 45 min in TBS-Tween, shaken with a 1:750 dilution of peroxidase-conjugated rabbit anti-mouse IgG (ICN Biomedicals Inc.) for 2 h and washed 3 times in TBS-Tween. Bound antibody was visualized by incubating blots in TBS containing chloronaphthol (Sigma, St. Louis, MO; 1 µg/ml) and 30% (v/v) hydrogen peroxide solution (2 µl/ml).
- Adhesion tests
Platelet TSP-1 was prepared as previously described (Adams and Lawler, 1994). Rat plasma fibronectin was obtained from Telios (San Diego, CA); human placenta collagen IV, rat plasma vitronectin, mouse EHS laminin (laminin-1; Wewer and Engvall, 1994), platelet factor 4, chondroitin sulfate A, heparin and β-xylosides were obtained from Sigma. Plasma fibronectin 40 kDa chymotryptic heparin-binding fragment and peptide GRGDSP were obtained from Life Technologies (Paisley, Scotland).
Cell-matrix adhesion Recombinants assays were performed in serum-free medium on glass coverslips coated as previously described (Adams and Lawler, 1994; Adams, 1995), typically for periods of 1 hour at 37°C. In experiments using a mixed substrate, glycoproteins were diluted to equimolar coating concentrations, mixed in different volume ratios, and used for coating. Soluble inhibitors were added at the time of seeding the cells. Non-adherent cells were removed by gentle washing in TBS containing 2 mM CaCl2, and adherent cells were fixed and processed for immunofluorescence.
- Confocal microscopy
Cells were stained as described above and examined by confocal microscopy, using a Leica DM-RBE and laser microscope. The images were acquired in the Leica TCS NT program. 32 or 45 serial optical sections were made throughout the entire depth of a cell using an image size of 512 × 512 pixels. Reconstruction of three-dimensional (3D) images was carried out using the NIH Image program.
- Time-lapse video microscopy
C2C12 cells plated at 3 × 10 4 cells/flask in Nunc Slide Flasks were monitored for periods of 4 h in an environmental chamber at 37 °C using a Zeiss Axiovert 100 microscope equipped with a Sony SS-M37OCE coupled device camera. switch connected to a video recorder and controlled by an EOS BAC900 animation controller. Cultures treated with 500 µg/ml heparin 2 h after plating were monitored between 4 h and 8 h after the start of treatment. Migration was recorded at 4 frames/min and the moving distances and velocities of individual cells were calculated from the tracks. At least 50 cells were screened for each experimental condition. Statistical significance was determined using a two-tailed t-test. Parallel cultures were stained for fascin.