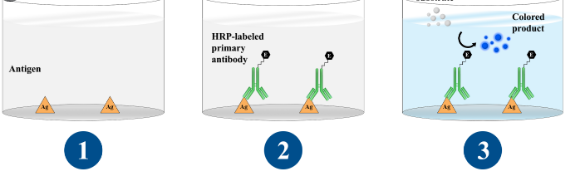
General process and suggestions for direct ELISA
Buffers and reagents:
Bicarbonate/carbonate coating buffer (100 mM)
Antigen or antibody must be diluted in coating buffer to immobilize them to the wells:
3.03 g Na2CO3
6.Zero g NaHCO3
1000 ml distilled water
pH 9.6
PBS:
1.16 g Na2HPO4,
0.1 g KCl
0.1 g K3PO4,
4.Zero g NaCl (500 ml distilled water) pH 7.4.
Blocking resolution:
Commonly used blocking brokers are 1% BSA , serum, non-fat dry milk, casein, gelatin in PBS.
Wash resolution:
Usually PBS or Tris-buffered saline (pH 7.4) with detergent similar to 0.05% (v/v) Tween20 (TBST).
Antibody dilution buffer:
Primary and secondary antibody must be diluted in 1x blocking resolution to scale back non particular binding.
General process:
Coating antigen to microplate
- Dilute the antigen to a remaining focus of 20 μg/ml in PBS or different carbonate buffer. Coat the wells of a PVC microtiter plate with the antigen by pipeting 50 μl of the antigen dilution within the prime wells of the plate. Dilute down the plate as required.
Test samples containing pure antigen are often pipeted onto the plate at lower than 2 μg/ml. Pure options aren’t important, however as a tenet, over 3% of the protein within the take a look at pattern must be the goal protein. (antigen). Antigen protein focus shouldn’t be over 20 μg/ml as it will saturate many of the obtainable websites on the microtitre plate.
Ensure the samples comprise the antigen at a focus that’s inside the detection vary of the antibody. - Cover the plate with an adhesive plastic and incubate for two h at room temperature, or 4°C in a single day. The coating incubation time might require some optimisation.
- Remove the coating resolution and wash the plate twice by filling the wells with 200 μl PBS. The options or washes are eliminated by flicking the plate over a sink. The remaining drops are eliminated by patting the plate on a paper towel.
Blocking
- Block the remaining protein-binding websites within the coated wells by including 200 μl blocking buffer, 5% non fats dry milk/PBS, per nicely. Alternative blocking reagents embody BlockACE or BSA.
- Cover the plate with an adhesive plastic and incubate for not less than 2 h at room temperature or, if extra handy, in a single day at 4°C.
- Wash the plate twice with PBS.
Incubation with the antibody
- Add 100 μl of the antibody, diluted on the optimum focus (in response to the producer’s directions) in blocking buffer instantly earlier than use.
- Cover the plate with an adhesive plastic and incubate for two h at room temperature. This incubation time might require optimisation. Although 2 hours is often sufficient to acquire a powerful sign, if a weak sign is obtained, stronger staining will usually noticed when incubated in a single day at 4°C.
- Wash the plate 4 instances with PBS.
Detection
- Dispense 100 μl (or 50 μl) of the substrate resolution per nicely with a multichannel pipet or a multipipet.
- After enough coloration improvement (whether it is vital) add 100 μl of cease resolution to the wells.
- Read the absorbance (optical density) of every nicely with a plate reader.
Note: some enzyme substrates are thought of hazardous (potential carcinogens), subsequently all the time deal with with care and put on gloves.
Analysis of knowledge:
Prepare an ordinary curve from the info produced from the serial dilutions with focus on the x axis (log scale) vs absorbance on the Y axis (linear). Interpolate the focus of the pattern from this commonplace curve.